So3 2 Lewis Structure
Na 2 SO 3 or sodium sulfite is a white solid inorganic compound with molar mass 126043 gmol. If you havent understood anything from the above image of SO3 sulfur trioxide lewis structure then just stick with me.

So3 Molecular Geometry Bond Angles Sulfur Trioxide Molecular Geometry Molecular Molecules
Let me explain this in detail with the help of SO3 2- lewis structure and its 3D geometry.

. Calculate the total number of valence electrons. Lets do the SO3 2- Lewis structure. For the SO3 2- Lewis structure the total number of valence electrons.
And the outside atoms oxygens also form an octet. Lets do the SO3 2- Lewis structure. In the above structure you can see that the central atom sulfur forms an octet.
Lewis proposed a basic technique of expressing valence electrons in order to. Chemical bonds are almost often created by valence electron interactions in atoms. The S atom is selected because it is present once and is the least electronegative atom.
The first step in composing a Lewis structure is to choose a center atom. Shown below is the Lewis structure of SO 3 2 _32- 3 2. For the SO3 2- compound we have 26 total valence electrons and that includes these two electrons up here--there are two extra valence electrons.
Trisulfide2- S3-2 CID 5460599 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. SO3 Lewis Structure. These ẟ and ẟ- charges are responsible to make the entire SO3 2- ion polar.
For the SO3 2- compound we have 26 total valence electrons and that includes these two electrons up here--there are two extra valence electrons. The electronegativity of an atom is defined as its ability to attract a shared pair of electrons from a covalent chemical bond. Here the given molecule is SO3 sulfur trioxide.
The sulfur trioxide SO3 is a tetra-atomic chemical molecule where three oxygen molecules and a sulfur bond have the same number of valence electrons. Let us discuss the structure and some important characteristics on Na 2 SO 3 briefly. In order to draw the lewis structure of SO3 first of all you have to find.
A quick explanation of the molecular geometry of SO3 2- Sulfite ion including a description of the SO3 2- bond anglesLooking at the SO3 2- Lewis structure. How to draw the Lewis Structure of SO3 sulfur trioxide - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electronsCheck. There are 2 lone pairs on all three Oxygen atoms O.
Find the least electronegative atom and place it at the center. A step-by-step explanation of how to draw the SO3 2- Lewis Structure Sulfite Ion. The structure shows two singly-bonded oxygen atoms and one doubly-bonded oxygen atom attached to a central sulfur atom.
There are 6 1 atom S 6. Why is SO3 2-. Hence the octet rule is satisfied.
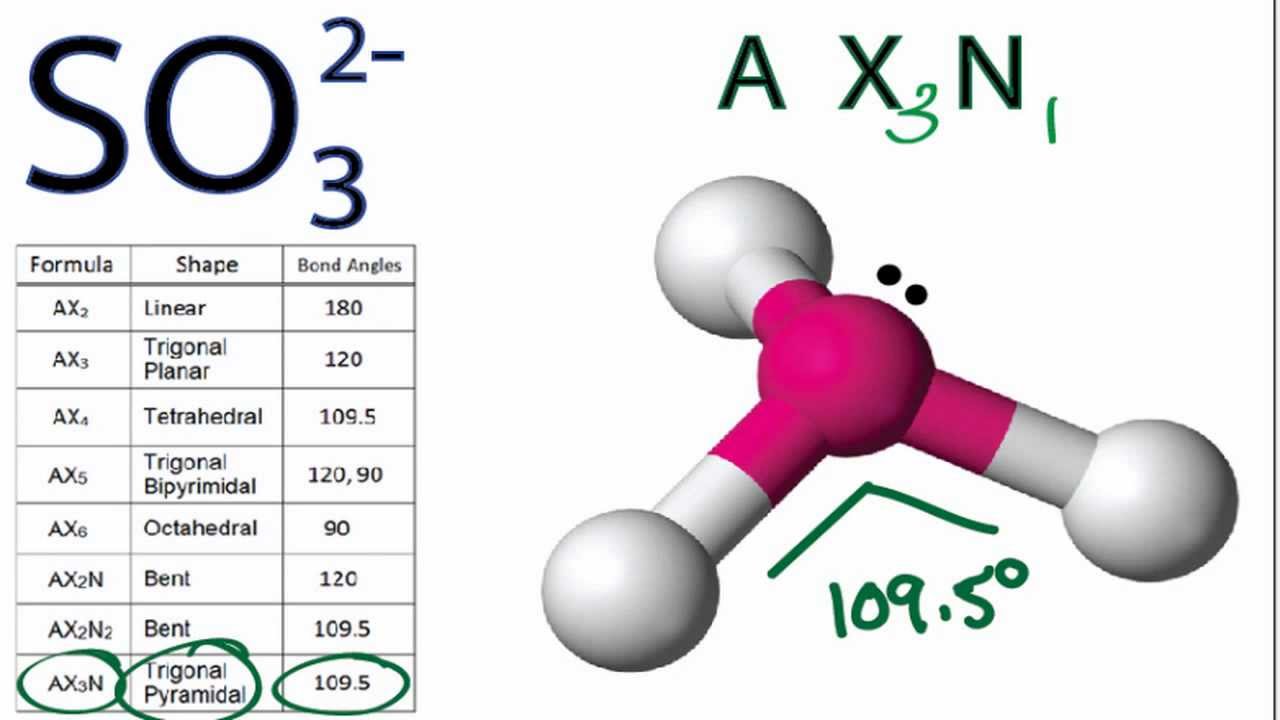
So32 Molecular Geometry Shape And Bond Angles Molecular Geometry Chemistry Help Molecular

Lewis Structure Of So3 Sulfur Trioxide Chemistry Worksheets Octet Rule Chemistry Notes

Lewis Structure Of So3 Sulfur Trioxide Exceptions To The Octet Rule So Tricky Lewis Structure Can Be Chemistry Education Octet Rule Free Science Lesson

Chemistry Chemical Bonding 18 Of 35 Lewis Structures For Ionic Compounds This Lecture By Michel Van Bie Chemistry Education Chemistry Worksheets Chemistry

So3 2 Lewis Structure Sulfite Ion Lewis Molecules Understanding

Lewis Structures Of Sulfur Trioxide So3 Electrostatic Potentials Esp Molecular Geometry Chemistry Lessons Chemistry Education
Comments
Post a Comment